Time:2021-12-03 Browse times:136
On November 26, 2021, Associate Professor Gong Hongri, Academician Rao Zihe and Associate Professor Gao Yan of the Institute of Immunochemistry of Shanghai University of Science and Technology and other research groups from the School of Life Sciences of Nankai University published a titled Structure of Mycobacterium tuberculosis cytochrome bcc in complex with Q203 and TB47, two anti-TB drug candidates in the internationally renowned academic journal eLifeResearch paper.Using single-particle cryo-electron microscopy, the team analyzed the natural state of mycobacteria mycobacteria respiratory chain cytomegic bcc complex and three high-resolution structures combined with Q203, which has completed clinical Phase II trials, and TB47, which are in preclinical trials, respectively.With the help of molecular dynamics and other technologies, the molecular mechanism of Q203 and TB47 to play a specific sterilization function is expounded for the first time, which will play a great role in further optimizing the above-mentioned candidate drugs and developing more effective anti-TB drugs.

Tuberculosis (Tuberculosis, TB) is an infectious disease caused by Mycobacterium tuberculosis.On October 14, 2021, the World Health Organization (WHO) released the Global Tuberculosis Report 2021. The WHO estimates that nearly 2 billion people are living with TB latent infection worldwide. In 2020, there will be 9.87 million new TB cases worldwide, and 842,000 new TB cases in China (833,000 in 2019).In order to control tuberculosis, there is an urgent need for new anti-TB drugs, especially those that can target multidrug-resistant and extremely resistant strains and that can shorten treatment time.The phosphate oxide (OXPHOS) system of Mycobacterium tuberculosis is necessary for the growth and survival of Mycobacterium tuberculosis, and is considered an important target for the development of new anti-TB drugs.Drugs targeting the system could lead to new treatments for drug-sensitive and drug-resistant tuberculosis, which pose a serious threat to human health.Telacebec, a new anti-tuberculosis candidate drug that is currently of concern and has completed clinical Phase II trials and achieved positive results, and TB47, which are in preclinical trials, achieve sterilization by inhibiting the function of mycobacteria mycobacteria respiratory cell pigment bcc complex. However, the molecular mechanisms for their specific inhibition function are not yet clear.
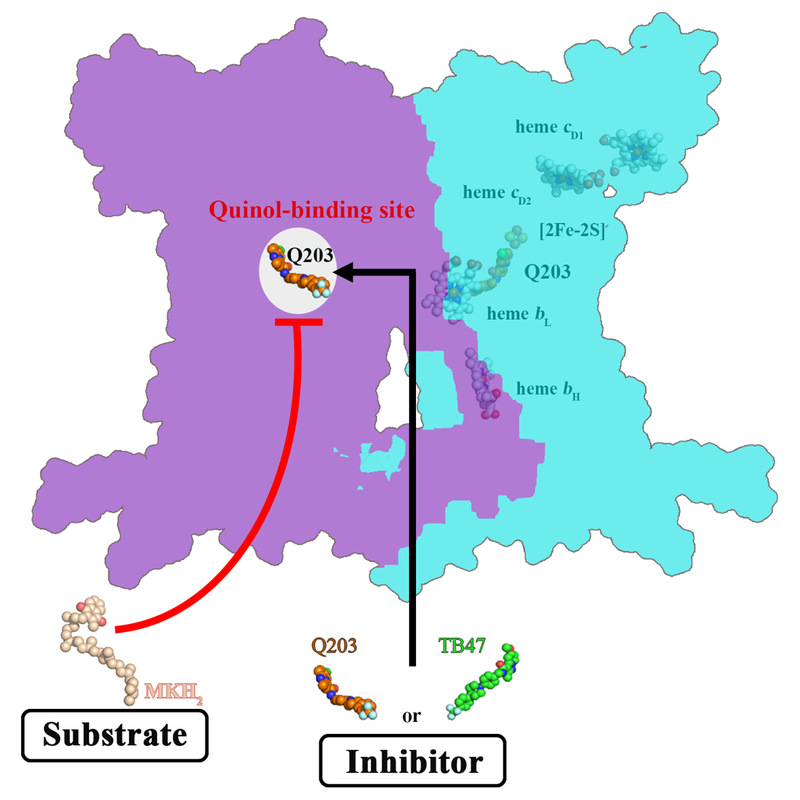
The mechanism of action of Q203 and TB47 targeting the cytochrome bcc complex of Mycobacterium tuberculosis
This study found that Q203 and TB47 bind to the oxidation site of the natural substrate quinone of the Mycobacterium tuberculosis respiratory chain cytochrome bcc complex, thereby preventing the binding of the electron-donating substrate quinone, and inhibiting the normal electron transfer. The transmission hinders the synthesis of adenosine triphosphate (ATP), which is the currency of energy circulation, thereby achieving the effect of starving the tuberculosis bacteria.
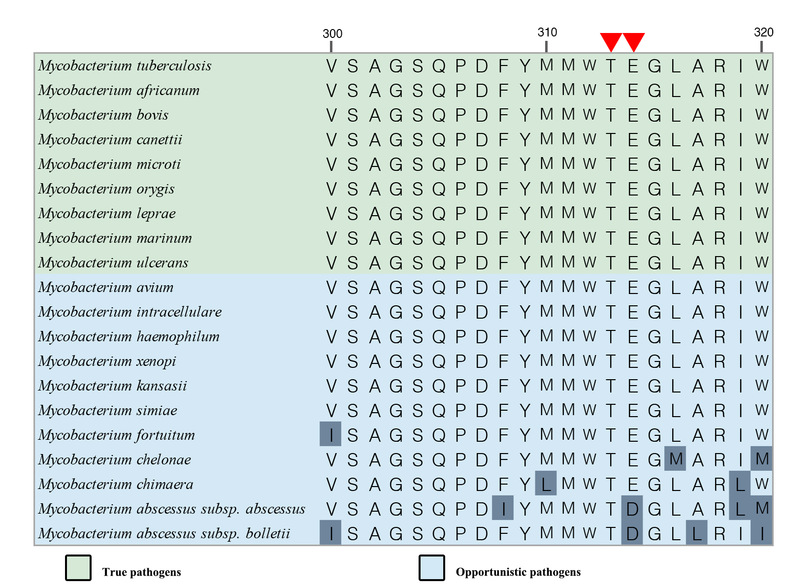
Amino acid sequence alignment of QcrB subunit of pathogenic mycobacterium cytochrome bcc complex
It is worth mentioning that, based on functional analysis, structural analysis and molecular dynamics simulations, the amino acid residues 313Thr and 314Glu (both are amino acid residues of the QcrB subunit) that play a key role in the stable binding of Q203 and TB47 due to the formation of hydrogen bonds ) Is highly conserved among pathogenic mycobacteria.Therefore, the results of this research will also help guide the application of Q203 and TB47 in the clinical treatment of other pathogenic mycobacteria and the development of new inhibitors.In addition, research reports that Q203 and TB47 have a good bactericidal effect on Mycobacterium ulcerans that cause Buruli ulcer disease in humans also further confirm the results of this research.
The first authors of this article are Zhou Shan, a PhD student in the School of Pharmacy, Nankai University, and Wang Weiwei, a PhD student in the School of Life Sciences, ShanghaiTech University. Tang Yanting, a postdoctoral fellow from the School of Life Sciences, and Lai Yuezheng, a 2021 Master and Ph.D. pass through exempt student participated in this research deeply.Associate Professor Gong Hongri, Academician Rao Zihe of Nankai University and Associate Researcher Gao Yan of Shanghai University of Science and Technology are the co-corresponding authors. Nankai University is the first completion unit.
This research was supported by the technical support of the Shanghai University of Science and Technology Bioelectron Microscopy Platform Center, the National Protein Science Center (Shanghai) Mass Spectrometry Platform, and the Ministry of Science and Technology and the National Natural Science Foundation of China.
Full text link: https://elifesciences.org/articles/69418